HepQuant Demonstrates New Advancements in Management of Patients with Acute Liver Failure Dependent on Extracorporeal Liver Cross-Circulation (ELC)
May 12, 2025Denver, CO – May 12, 2025 – HepQuant, LLC released exciting data about an innovative application of liver function quantification that may aid in future patient management decisions for patients with acute or acute on chronic liver failure who are dependent on an extracorporeal liver cross-circulation (ELC). Dr. Greg T. Everson, HepQuant’s CEO and founder, presented oral and poster presentations at DDW 2025 and EASL Congress 2025 to bring these unique findings to hepatology thought leaders. Specifically, the 13C-cholate method was used to simultaneously quantify the function of native human liver and EGEN-5784 porcine liver in a decedent study of the eGenesis liver in combination with the OrganOx ELC system.
More than one in ten Americans has liver disease, with approximately 35,000 that are diagnosed with acute or acute on chronic liver failure each year.1,2 Current treatment options for these patients are limited, resulting in overall mortality exceeding 50% annually.2 HepQuant has collaborated with eGenesis and Dr. Abraham Shaked of University of Pennsylvania in a pilot study to evaluate measurements of both human liver and the eGenesis human-compatible, genetically modified porcine liver (EGEN-5784). eGenesis and OrganOx recently announced the FDA clearance of an investigational new drug application for EGEN-5784 used in combination with the OrganOx ELC system to support patients with acute-on-chronic liver failure.3
HepQuant’s technology includes liver cell specific, flow-dependent measurements of intravenous13C-cholate clearance to assess liver health and physiology. The pilot study design included four brain-dead human decedents that were evaluated prior to and during the use of the ELC system using genetically modified porcine livers. Blood samples were collected from human decedents at specific timepoints following administration of 13C-cholate. At the same timepoints, porcine liver and ELC system inflow and outflow were also measured. Data was then analyzed from all samples to measure cholate clearance in both human and porcine livers to evaluate the resulting changes to liver function.
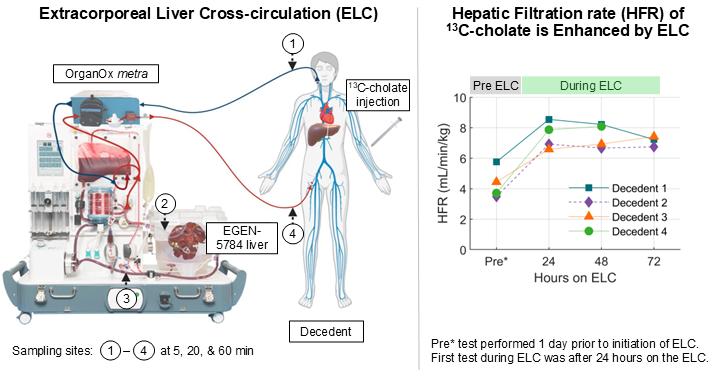
Reported study results and conclusions highlighted the reproducibility of measured clearance (r2=0.9616) at each element of an ELC and demonstrated the enhancement of cholate clearance when an ELC was incorporated into care (above graph). This technology may aid in future management decisions for patients with acute or acute on chronic liver failure who are dependent on ELC systems while awaiting transplant. Dr. Gregory Everson, CEO of HepQuant, shared: “We are excited to continue our collaborative work with the 13C-cholate method to facilitate real-time simultaneous measurement of the liver function of both patient liver and extracorporeal porcine liver to support critical care decisions for patients with acute liver failure.”
References:
- American Liver Association Facts about Liver Disease, org. Updated September 11, 2023.
- Allen, A.M., et al. (2016). Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology, 64(6), pp. 2165–2172. https://doi.org/10.1002/hep.28812.
- eGenesis (2025, Apr 15). eGenesis-and-OrganOx-Announce-U.S.-FDA-Clearance-of-IND-Application-for-the-Treatment-of-Patients-with-Acute-On-Chronic-Liver-Failure. https://eGENESISbio.com/press-releases/eGENESIS-and-organox-announce-u-s-fda-clearance-of-ind-application-for-the-treatment-of-patients-with-acute-on-chronic-liver-failure/.
About HepQuant
HepQuant has developed noninvasive, blood-based, quantitative tests that assess liver health by measuring critical liver cell processes and blood flow to the liver. Our test results, in conjunction with other clinical assessments, inform healthcare providers’ clinical decisions to achieve more effective management of patients with advanced liver disease. Knowing where a patient falls on the disease spectrum informs personalized treatment decisions for that individual. HepQuant is a privately held diagnostics company based in Denver, Colorado. Learn more at HepQuant.com.
HepQuant DuO is a Laboratory Developed Test (LDT). This test was developed and its performance characteristics determined by HepQuant, LLC in a manner consistent with CLIA requirements. This test has not been cleared or approved by the U.S. Food and Drug Administration
Contact
Joellyn Enos, Chief Commercial Officer
Joellyn.Enos@HepQuant.com
303.268.7069