Innovation with a Purpose
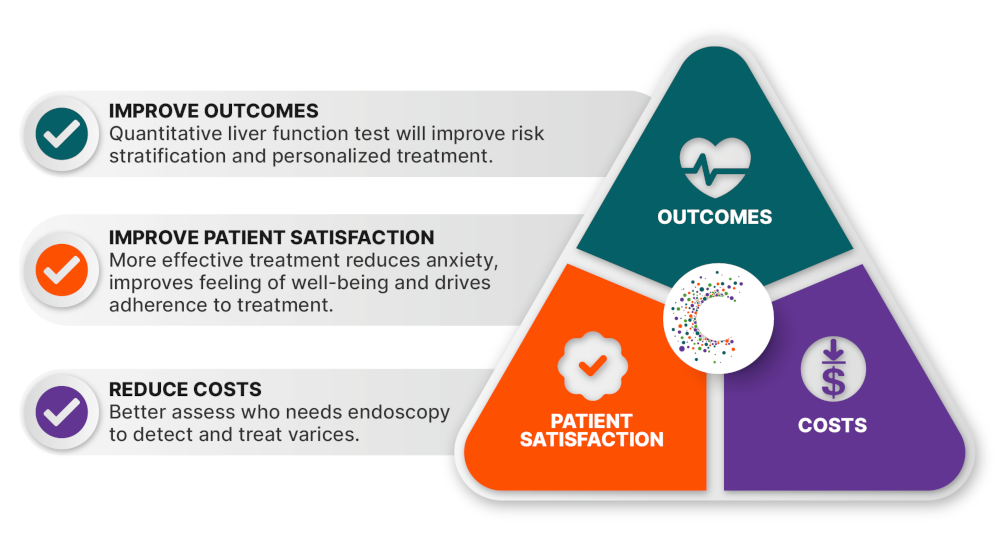
Any new medical device needs to satisfy an unmet need or fill a clinical gap to be successful. More than ever before, healthcare innovators must align with the “Triple Aim” shared by providers and payors: Improve health outcomes, enhance patient satisfaction and reduce costs.
At HepQuant, we strive to meet the Triple Aim challenge by delivering actionable results that inform treatment decisions to improve the lives of patients, their families and the providers who care for them.
Medical Need
The Problem
Today’s diagnostic toolbox does not have a quantitative liver function test. The HepQuant DuO Test fulfills this unmet need.
Our Solution
HepQuant tests measure uptake of cholate by the liver to quantitate liver function and portal systemic shunting. Cholate uptake is a natural process that is specific to the liver. Results may be used to help assess and monitor liver function and provide actionable insights to inform clinical management.
MEASURE LIVER FUNCTION
Indicator of liver health: How well does the liver filter blood?
INFORM TREATMENT
Does the patient need endoscopy urgently to detect and treat varices?
IMPROVE OUTCOMES
Personalized treatment plan. Reduce unnecessary procedures.
Why has this not been done before?
It’s a very difficult technical challenge! HepQuant tests harness the power of advanced mass spectrometry, a highly sensitive analytical tool that measures ultra-low concentrations of molecules accurately. The HepQuant team developed the technology over many years and have evaluated the technology in multiple analytical and clinical studies. Please visit the Resources page to browse the publications and posters highlighting clinical data.
Learn more about HepQuant DuO and Pharma Solutions
The HepQuant DuO test is a quantitative liver function test that measures cholate clearance to assess liver function.
HepQuant works with pharma companies and academic researchers to test levels of hepatic impairment in clinical trials.
HepQuant DuO is a Laboratory Developed Test (LDT). This test was developed and its performance characteristics determined by HepQuant, LLC in a manner consistent with CLIA requirements. This test has not been cleared or approved by the U.S. Food and Drug Administration.
The HepQuant DuO Test is not available for sale. It will be available soon to healthcare professionals as an adjunctive aid in assessment of liver function, in conjunction with clinical evaluation and other tests.