PRNewswire-PRWeb January 12, 2026
2026 MASH-TAG Oral Presentation: HepQuant DuO® Reveals Functional and Physiological Heterogeneity to Support Enhanced Clinical Trial Design
“HepQuant DuO is the ideal noninvasive test of liver health with clinical validation data confirming effective characterization of disease severity, tracking of disease progression and treatment impact.”, stated Dr. Gregory T. Everson, Chief Executive Officer of HepQuant. “HepQuant is committed to bringing this important quantitative tool to healthcare providers to transform liver health and improve patient management.”
PRNewswire-PRWebNovember 6, 2025
HepQuant Presents Exciting Abstracts at The Liver Meeting 2025 Demonstrating HepQuant DuO® Utility to Uncover Functional and Physiological Heterogeneity
HepQuant, a leader in developing noninvasive, blood-based, quantitative testing to assess liver health, will present three exciting abstracts demonstrating HepQuant DuO utility at The Liver Meeting 2025® conference this week. Each demonstrates how HepQuant DuO test results uncover functional and physiological heterogeneity in patients with advanced MASH and chronic HCV (Child Pugh A5 compared to Child Pugh A6). The third abstract presentation utilizes the HepQuant DuO test to measure the recovery of function in living donors who underwent right lobectomy.
PRNewswire-PRWeb October 14, 2025
HepQuant Announces Exciting New Data Utilizing HepQuant DuO® testing: Early Assessment of Resmetirom Treatment Benefit in Patients with Compensated MASH Cirrhosis
HepQuant, a leader in developing noninvasive, blood-based, quantitative testing to assess liver health, announces promising findings from a study utilizing the HepQuant DuO test to evaluate the impact of resmetirom treatment in patients with metabolic dysfunction-associated steatohepatitis (MASH)-related Child-Pugh A cirrhosis. In the peer-reviewed publication, 83% of patients showed either stability or improvement in liver function, as measured by DSI, after 48 weeks.
PRNewswire-PRWebOctober 2, 2025
HepQuant Presents a New Application for the HepQuant DuO™ Test: Improved Study Design and Treatment Monitoring in Clinical Trials
HepQuant, a leader in developing noninvasive, blood-based, quantitative testing to assess liver health, presented promising data utilizing the HepQuant DuO™ test to quantify liver function and physiology at an oral presentation on September 30, 2025 during the 9th Annual MASH Drug Development Summit in Boston, MA. Dr. Gregory T. Everson shared exciting data on the use of the HepQuant DuO test as a reasonably likely surrogate endpoint for clinical trials, highlighting this valuable tool in evaluating patients and planning studies.
PRNewswire-PRWebJune 2, 2025
HepQuant Achieves ISO 13485 Certification to Support Scaling of HepQuant DuO™ to Global Clinical Trials
HepQuant, LLC, a leader in developing noninvasive, blood-based, quantitative testing to assess liver health, is proud to announce ISO 13485 certification as its latest milestone in operational excellence. This internationally recognized certification is a significant achievement that underscores the company's commitment to the highest standards in quality management and regulatory compliance. This milestone enhances HepQuant's abilities to engage in larger, more impactful clinical studies to drive transformative advancements to improve liver health.
May 12, 2025
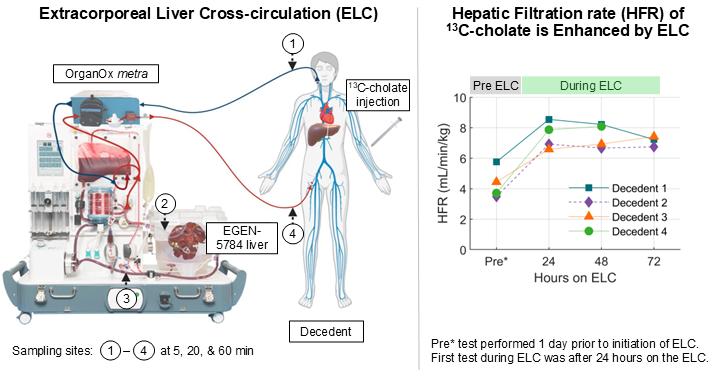
HepQuant Demonstrates New Advancements in Management of Patients with Acute Liver Failure Dependent on Extracorporeal Liver Cross-Circulation (ELC)
Simultaneous evaluation of Human and Porcine liver functions using 13C-Cholate quantification may aid in future management of patients with acute liver failure who are assisted by ELC systems while awaiting transplant.
April 30, 2025
HepQuant to Present Quantification of Human and Porcine Liver Function in an Extracorporeal Liver Circuit (ELC) at the DDW 2025 Late-Breaking Plenary Session
Dr. Greg T. Everson will discuss the results of studies with 13C-cholate for liver function measurement for this application on May 3, 2025, from 2:00 to 3:00 pm at the San Diego Convention Center
March 17, 2025
HepQuant to Present at Key Liver Conferences to Highlight Applications of HepQuant DuO™ – a Quantitative Liver Health Test
Recent clinical data demonstrates how HepQuant DuO test results may be used to inform endoscopy screening decisions, monitor disease progression and evaluate response to resmetirom therapy in patients with compensated advanced chronic liver disease (cACLD). Denver, CO – March 17, 2025 – HepQuant, LLC continues to expand clinical availability of…
June 13, 2023
HepQuant Announces Additions to the Executive Leadership Team to Drive Commercialization
Kelly R. Pitts, PhD, Chief Operating Officer; Carrie Mulherin, MBA, Chief Commercial Officer; Shailesh Chavan, MD, Chief Medical Officer; Paige Nardi, MBA VP Market Access Denver, CO – June 13, 2023 – HepQuant, LLC, a leader transforming the management of liver diseases, announces key additions to its executive leadership team….