HepQuant Achieves ISO 13485 Certification to Support Scaling of HepQuant DuO™ to Global Clinical Trials
HepQuant, LLC, a leader in developing noninvasive, blood-based, quantitative testing to assess liver health, is proud to announce ISO 13485 certification as its latest milestone in operational excellence. This internationally recognized certification is a significant achievement that underscores the company's commitment to the highest standards in quality management and regulatory compliance. This milestone enhances HepQuant's abilities to engage in larger, more impactful clinical studies to drive transformative advancements to improve liver health.HepQuant Demonstrates New Advancements in Management of Patients with Acute Liver Failure Dependent on Extracorporeal Liver Cross-Circulation (ELC)
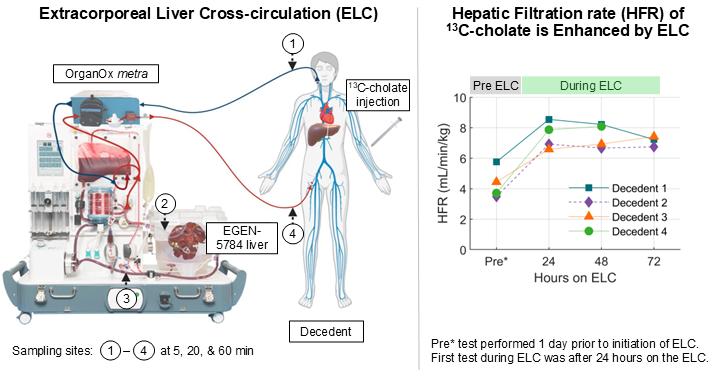
Simultaneous evaluation of Human and Porcine liver functions using 13C-Cholate quantification may aid in future management of patients with acute liver failure who are assisted by ELC systems while awaiting transplant.HepQuant to Present Quantification of Human and Porcine Liver Function in an Extracorporeal Liver Circuit (ELC) at the DDW 2025 Late-Breaking Plenary Session
Dr. Greg T. Everson will discuss the results of studies with 13C-cholate for liver function measurement for this application on May 3, 2025, from 2:00 to 3:00 pm at the San Diego Convention CenterHepQuant to Present at Key Liver Conferences to Highlight Applications of HepQuant DuO™ – a Quantitative Liver Health Test
Recent clinical data demonstrates how HepQuant DuO test results may be used to inform endoscopy screening decisions, monitor disease progression and evaluate response to resmetirom therapy in patients with compensated advanced chronic liver disease (cACLD). Denver, CO – March 17, 2025 – HepQuant, LLC continues to expand clinical availability of…
HepQuant Announces Additions to the Executive Leadership Team to Drive Commercialization
Kelly R. Pitts, PhD, Chief Operating Officer; Carrie Mulherin, MBA, Chief Commercial Officer; Shailesh Chavan, MD, Chief Medical Officer; Paige Nardi, MBA VP Market Access Denver, CO – June 13, 2023 – HepQuant, LLC, a leader transforming the management of liver diseases, announces key additions to its executive leadership team….
Kelly R. Pitts, Ph.D., Appointed Chief Operating Officer
Medical Diagnostics Industry Veteran with Biotech and Biopharma Experience Will Guide Operations DENVER (Apr. 14, 2023) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced its has chosen Kelly R. Pitts, Ph.D., as its new Chief…
HepQuant Lab Moves to New Location Within Fitzsimons Innovation Community Campus
The HepQuant Lab has moved a short distance within the Fitzsimons Innovation Community Campus to Bioscience 1, located at 12635 E. Montview Blvd., Suite 175, Aurora, CO 80045. The new lab space is adjacent to our previous location where we’ve been for the past five years, according to HepQuant Chief…