News and Events
2025 Texas Society of Gastroenterology and Endoscopy Annual Meeting
HepQuant Achieves ISO 13485 Certification to Support Scaling of HepQuant DuO™ to Global Clinical Trials
HepQuant, LLC, a leader in developing noninvasive, blood-based, quantitative testing to assess liver health, is proud to announce ISO 13485 certification as its latest milestone in operational excellence. This internationally recognized certification is a significant achievement that underscores the company's commitment to the highest standards in quality management and regulatory compliance. This milestone enhances HepQuant's abilities to engage in larger, more impactful clinical studies to drive transformative advancements to improve liver health.HepQuant Demonstrates New Advancements in Management of Patients with Acute Liver Failure Dependent on Extracorporeal Liver Cross-Circulation (ELC)
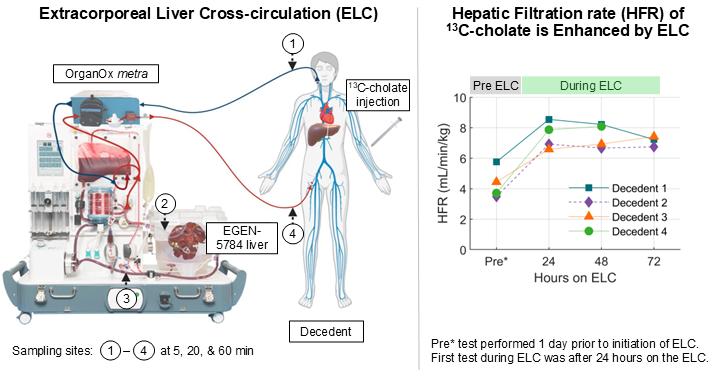
Simultaneous evaluation of Human and Porcine liver functions using 13C-Cholate quantification may aid in future management of patients with acute liver failure who are assisted by ELC systems while awaiting transplant.HepQuant to Present Quantification of Human and Porcine Liver Function in an Extracorporeal Liver Circuit (ELC) at the DDW 2025 Late-Breaking Plenary Session
Dr. Greg T. Everson will discuss the results of studies with 13C-cholate for liver function measurement for this application on May 3, 2025, from 2:00 to 3:00 pm at the San Diego Convention CenterHepQuant Celebrates Lab Week 2025!
Our amazing lab team is the backbone of our company, powering innovation, precision, and progress every single day. Their dedication to science, quality, and patient impact makes everything we do possible. This week, we recognize and thank our lab professionals for their hard work behind the scenes—advancing liver disease diagnostics and helping improve lives. 🔬 Here’s to the problem solvers, data champions, and science heroes in the lab. We appreciate you today and every day!NAMCP’s Spring Managed Care Forum
HepQuant’s Paige Nardi (VP, Market Access, Billing & Reimbursement) and Kerry Whitaker (Senior Medical Science Liaison) will be attending NAMCP’s Spring Managed Care Forum on April 24–25 at Rosen Creek, Orlando, FL. Stop by Booth 216 to meet with them!
EASL Congress 2025
HepQuant’s own Dr. Gregory T. Everson, CEO will present two posters at EASL Congress 2025 in Amsterdam, the Netherlands May 7 – 10. Top Poster #507 “Quantifying liver function by cholate clearance in extracorporeal circuits with a genetically modified porcine liver and brain-dead human decedent” and poster #355 “The HepQuant…
DDW 2025
Dr. Gregory T. Everson, CEO and founder of HepQuant, will discuss “Simultaneous Quantification of Native Human Liver and Porcine Liver Function in an Extracorporeal Circuit Using IV 13C-Cholate.” at the upcoming DDW 2025 Late-Breaking Plenary session on May 3, 2025. Please join him in Room 25 from 2:00 to 3:00 pm…
Global Hepatitis Summit 2025
HepQuant’s Dr. Joanne Imperial, Chief Medical Officer, will be attending the Global Hepatitis Summit 2025 in Los Angeles and presenting a poster titled HepQuant DuO™ for Quantifying Liver Function and Physiology in the Clinic: Diagnostic Performance for Esophageal Varices in Patients with Treated and Active Chronic Hepatitis C, poster P-187.
5th Annual Liver Connect Conference
HepQuant will participate in the 5th Annual Liver Connect Conference in San Antonio, TX. A silver sponsor of the event, the HepQuant team may be found at booth 17 in the exhibit hall. Three posters submitted by HepQuant will be featured during the conference. Poster 47: Initial Clinical Experience with…
HepQuant to Present at Key Liver Conferences to Highlight Applications of HepQuant DuO™ – a Quantitative Liver Health Test
Recent clinical data demonstrates how HepQuant DuO test results may be used to inform endoscopy screening decisions, monitor disease progression and evaluate response to resmetirom therapy in patients with compensated advanced chronic liver disease (cACLD). Denver, CO – March 17, 2025 – HepQuant, LLC continues to expand clinical availability of…
AASLD, The Liver Meeting
HepQuant is excited that six abstracts, will be presented, three of which were deemed a Poster of Distinction, at The Liver Meeting 2024 on 11/16/24 and 11/17/24 linked below. Poster of Distinction: The Oral Cholate Challenge Test Defines Likelihood of Large Esophageal Varices in an Overweight and Obese US Population …
HepQuant Paticipates in Media Pitch Day
Fitzsimmons Innovation Community hosted its first Media Pitch Day. Check out the summary:HepQuant Announces Additions to the Executive Leadership Team to Drive Commercialization
Kelly R. Pitts, PhD, Chief Operating Officer; Carrie Mulherin, MBA, Chief Commercial Officer; Shailesh Chavan, MD, Chief Medical Officer; Paige Nardi, MBA VP Market Access Denver, CO – June 13, 2023 – HepQuant, LLC, a leader transforming the management of liver diseases, announces key additions to its executive leadership team….
“As it awaits key readout, Hepion boosts confidence in NASH drug hopeful via shorter study”
Kelly R. Pitts, Ph.D., Appointed Chief Operating Officer
Medical Diagnostics Industry Veteran with Biotech and Biopharma Experience Will Guide Operations DENVER (Apr. 14, 2023) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced its has chosen Kelly R. Pitts, Ph.D., as its new Chief…
HepQuant Lab Moves to New Location Within Fitzsimons Innovation Community Campus
The HepQuant Lab has moved a short distance within the Fitzsimons Innovation Community Campus to Bioscience 1, located at 12635 E. Montview Blvd., Suite 175, Aurora, CO 80045. The new lab space is adjacent to our previous location where we’ve been for the past five years, according to HepQuant Chief…
Hepion Pharmaceuticals Announces Early Completion of Enrollment in Phase 2 ‘ALTITUDE-NASH’ Liver Function Trial
Hepion Pharma Announces First Dosing in Phase 2b Clinical Trial with HepQuant
Hepion Pharma Announces First Dosing in Phase 2b Clinical Trial with HepQuant Endpoints
HepQuant Lab Meets COLA – CLIA Quality Standards
DENVER (Aug. 24, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that the HepQuant laboratory has achieved COLA accreditation for laboratory quality assurance. COLA is a third-party accreditation organization that ensures labs comply with federal…
Preliminary data from the HepQuant SHUNT-V Pivotal study presented at DDW® 2022
SAN DIEGO (May 24, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced preliminary data from the SHUNT-V Pivotal study in oral and poster presentations at Digestive Disease Week® (DDW) 2022 in San Diego….
Chris Jensen Joins Business Advisory Board
Former J.P. Morgan Investment Banker Brings Mid-Market Capital-Raising Experience to Board DENVER (May 10, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that former J.P. Morgan Investment Banker Chris Jensen has…
Bobbi Coffin Named to Business Advisory Board
Biodesix Exec and Former Exact Sciences Commercial Leader Adds Marketing Depth to Board Denver, CO – May 5, 2022 – HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that Bobbi Coffin, has joined the company’s…
Hepion Pharmaceuticals Announces Clinical Collaboration with HepQuant in Phase 2b NASH Trial
Statins Show Promise for Advanced Liver Failure and Cirrhosis
Pharma and Medical Device Veteran Ally Xu Named Director of Regulatory and Quality Affairs
HepQuant Names Pharma and Medical Device Veteran Ally Xu Director of Regulatory and Quality Affairs DENVER (Feb. 1, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that pharmaceutical and medical device industry veteran…
Preliminary data from the HepQuant SHUNT-V Pivotal study released during the 72nd AASLD Annual Meeting
HepQuant Results Presented while Attending the 72nd AASLD Annual Meeting and AHA 2021 Annual Meeting DENVER (November 16th, 2021) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that preliminary data from the SHUNT-V Pivotal study…
Perspectum and HepQuant Announce Partnership to Bring a More Complete Picture of Liver Health
Perspectum and HepQuant Announce Partnership to Bring a More Complete Picture of Liver Health Partnership Combines Imaging and Functional Assessment for Clinical Research San Francisco, CA and Denver, CO – Nov. 1, 2021. Perspectum and HepQuant are proud to announce a strategic business partnership to provide seamless delivery of HepQuant’s…
HepQuant’s Greg Everson, MD, to Speak at AASLD/FDA Conference on Drug-Induced Liver Injury (DILI)
HepQuant’s Greg Everson, MD, to Speak at AASLD/FDA Conference on Drug-Induced Liver Injury (DILI) DENVER (April 14th, 2021) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that it will present at the 2021 AASLD/FDA Conference…
HepQuant Promotes Sean Bundy to Chief Operating Officer
HepQuant Promotes Sean Bundy, RAC, to Chief Operating Officer Denver, CO – June 10, 2020 – HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced the promotion of Sean Bundy, RAC, to the…
HepQuant to Offer Mobile and Remote Test Administration via CVS Health’s Coram Clinical Trials
HepQuant to Offer Mobile and Remote Test Administration in Clinical Trials via CVS Health’s Coram Clinical Trials DENVER (February 18th, 2020) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending blood-based technology for evaluating the function of the liver, today announced that it will now offer…
HepQuant to Present at Biotech Showcase™ 2020
HepQuant to Present at Biotech Showcase™ 2020 San Francisco Event to be held January 13–15th DENVER (Jan. 10, 2020) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending blood-based technology for evaluating the function of the liver, today announced that it has scheduled a company presentation at…
HepQuant’s Disease Severity Index (DSI) Demonstrates Hepatic Functional Improvement in High Percentage of NASH Patients Treated with Obeticholic Acid: Results from INTERCEPT 747-117
HepQuant’s Disease Severity Index (DSI) Demonstrates Hepatic Functional Improvement in High Percentage of NASH Patients Treated with Obeticholic Acid: Results from INTERCEPT 747-117 The First HepQuant SHUNT™ Data From An Interventional Drug Study in NASH DENVER (April 4, 2019) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique,…
Hoffman Joins HepQuant as Clinical Lab Manager
Keith Hoffman Joins HepQuant as Clinical Lab Manager Brings more than 20 years of pharmaceutical industry experience in LC/MS analytics and lab management Keith Hoffman DENVER (Feb. 20, 2019) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients…
First Patient Enrolled in a Pivotal Trial of the HepQuant SHUNT Liver Diagnostic Test
First Patient Enrolled in a Pivotal Trial of the HepQuant SHUNT Liver Diagnostic Test Study Aims to Enroll 420 Subjects in 20 U.S. Research Sites DENVER (Feb. 14, 2019) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients…
Sarah Downing Joins HepQuant as Senior Manager of Quality Affairs
Sarah Downing Joins HepQuant as Senior Manager of Quality Affairs Certified Quality Engineer Brings More than 14 Years Experience in Medical Device and Diagnostics Quality Affairs Sarah Downing, Sr. Manager of Quality Affairs DENVER (Nov. 15, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending…
Receives IDE Approval from U.S. FDA to Initiate Clinical Trial of the SHUNT Liver Diagnostic Kit
HepQuant Receives IDE Approval from U.S. FDA to Initiate Clinical Trial of the SHUNT Liver Diagnostic Kit SAN FRANCISCO (Nov. 9, 2018) – While attending The Liver Meeting® 2018, HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic…
Sean Bundy Appointed Director of Regulatory and Quality Affairs
HepQuant Appoints Sean Bundy Director of Regulatory & Quality Affairs Bringing 20 Years in Medical Device and Diagnostics Regulatory and Quality Experience DENVER (Aug. 2, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease,…
HepQuant Chosen to Advance to InnoSTARS Semifinals in China
HepQuant Selected as a Semifinalist for 2018 InnoSTARS Competition to be Held in China DENVER (May 9, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, announced today that the company has been named…
HepQuant a Finalist for InnoSTARS Denver Competition
HepQuant a Finalist for 2018 InnoSTARS Denver Competition DENVER (April 24, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, announced today that the company has been named a finalist for the 2018 InnoSTARS Denver…
HepQuant and CTI Clinical Trial and Consulting Services Announce First Patient Enrolled in Liver Disease Diagnostic Trial
HepQuant and CTI Clinical Trial and Consulting Services Announce First Patient Enrolled in Liver Disease Diagnostic Trial FOR IMMEDIATE RELEASE [Covington, KY ~ January 25, 2018] HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease…
HepQuant to Present Poster as Finalist at RESI San Francisco Innovation Challenge
HepQuant to Present Poster as a Finalist in the RESI San Francisco Innovation Challenge 2018 DENVER (January 2nd, 2017) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced it will present a…
HepQuant to Present at the 10th Annual Biotech Showcase
HepQuant to Present at 10th Annual Biotech Showcase Senior Executives Will Also Attend the J.P. Morgan Healthcare Conference DENVER (December 13th, 2017) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced it…
HepQuant Presents at Rocky Mountain Life Science Investor and Partnering Conference
HepQuant Presents at the 2017 Rocky Mountain Life Science Investor and Partnering Conference DENVER (October 12th, 2017) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced it presented at the 2017 Rocky Mountain…
HepQuant holding its 2017 Scientific Advisory Board Meeting in Washington D.C. on Saturday, October 21st
HepQuant to Hold 2017 Scientific Advisory Board October 21st in Washington, DC DENVER (August 31, 2017) — HepQuant, LLC, a diagnostics company, located in Greenwood Village, CO with unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced today announced it will be holding…
HepQuant to Sponsor its First Clinical Study
HepQuant to Sponsor its First Clinical Study Utilizing 3 Investigational Device Exemptions (IDEs) in Clinical Study of Patients with Alcoholic Hepatitis, NASH F3, NASH F4 DENVER (Sep. 28, 2017) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients…
HepQuant Announces Approval of 4th IDE
HepQuant Announces Approval of Fourth Investigational Device Exemption Application HepQuant SHUNT™ Liver Diagnostic Kit for use in a clinical study in subjects with Alcoholic Hepatitis DENVER (September 14, 2017) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with…
HepQuant Completes Third State-Sponsored Grant
HepQuant Completes Third State-Sponsored Grant State of Colorado funding enables advancement of supply chain, regulatory advancements and HepQuant laboratory DENVER (August 31, 2017) — HepQuant, LLC, a diagnostics company, located in Greenwood Village, CO with unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease,…
Test Inventor and Founder Dr. Everson Accepts Role as CEO
HepQuant Test Inventor and Founder Doctor Gregory T. Everson Accepts Position of CEO DENVER (July 1, 2017) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that its liver test inventor, Dr. Greg…
Dr. Steve Helmke Appointed Chief Scientific Officer
HepQuant Announces Approval of 3rd IDE
HepQuant Announces Approval of 2nd IDE
HepQuant Announces Approval of 1st IDE
HepQuant Celebrates Lab Week 2025!
Our amazing lab team is the backbone of our company, powering innovation, precision, and progress every single day. Their dedication to science, quality, and patient impact makes everything we do possible. This week, we recognize and thank our lab professionals for their hard work behind the scenes—advancing liver disease diagnostics and helping improve lives. 🔬 Here’s to the problem solvers, data champions, and science heroes in the lab. We appreciate you today and every day!HepQuant Achieves ISO 13485 Certification to Support Scaling of HepQuant DuO™ to Global Clinical Trials
HepQuant, LLC, a leader in developing noninvasive, blood-based, quantitative testing to assess liver health, is proud to announce ISO 13485 certification as its latest milestone in operational excellence. This internationally recognized certification is a significant achievement that underscores the company's commitment to the highest standards in quality management and regulatory compliance. This milestone enhances HepQuant's abilities to engage in larger, more impactful clinical studies to drive transformative advancements to improve liver health.HepQuant Demonstrates New Advancements in Management of Patients with Acute Liver Failure Dependent on Extracorporeal Liver Cross-Circulation (ELC)
Simultaneous evaluation of Human and Porcine liver functions using 13C-Cholate quantification may aid in future management of patients with acute liver failure who are assisted by ELC systems while awaiting transplant.HepQuant to Present Quantification of Human and Porcine Liver Function in an Extracorporeal Liver Circuit (ELC) at the DDW 2025 Late-Breaking Plenary Session
Dr. Greg T. Everson will discuss the results of studies with 13C-cholate for liver function measurement for this application on May 3, 2025, from 2:00 to 3:00 pm at the San Diego Convention CenterHepQuant to Present at Key Liver Conferences to Highlight Applications of HepQuant DuO™ – a Quantitative Liver Health Test
Recent clinical data demonstrates how HepQuant DuO test results may be used to inform endoscopy screening decisions, monitor disease progression and evaluate response to resmetirom therapy in patients with compensated advanced chronic liver disease (cACLD). Denver, CO – March 17, 2025 – HepQuant, LLC continues to expand clinical availability of…
HepQuant Announces Additions to the Executive Leadership Team to Drive Commercialization
Kelly R. Pitts, PhD, Chief Operating Officer; Carrie Mulherin, MBA, Chief Commercial Officer; Shailesh Chavan, MD, Chief Medical Officer; Paige Nardi, MBA VP Market Access Denver, CO – June 13, 2023 – HepQuant, LLC, a leader transforming the management of liver diseases, announces key additions to its executive leadership team….
Kelly R. Pitts, Ph.D., Appointed Chief Operating Officer
Medical Diagnostics Industry Veteran with Biotech and Biopharma Experience Will Guide Operations DENVER (Apr. 14, 2023) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced its has chosen Kelly R. Pitts, Ph.D., as its new Chief…
HepQuant Lab Moves to New Location Within Fitzsimons Innovation Community Campus
The HepQuant Lab has moved a short distance within the Fitzsimons Innovation Community Campus to Bioscience 1, located at 12635 E. Montview Blvd., Suite 175, Aurora, CO 80045. The new lab space is adjacent to our previous location where we’ve been for the past five years, according to HepQuant Chief…
Hepion Pharmaceuticals Announces Early Completion of Enrollment in Phase 2 ‘ALTITUDE-NASH’ Liver Function Trial
Hepion Pharma Announces First Dosing in Phase 2b Clinical Trial with HepQuant Endpoints
HepQuant Lab Meets COLA – CLIA Quality Standards
DENVER (Aug. 24, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that the HepQuant laboratory has achieved COLA accreditation for laboratory quality assurance. COLA is a third-party accreditation organization that ensures labs comply with federal…
Preliminary data from the HepQuant SHUNT-V Pivotal study presented at DDW® 2022
SAN DIEGO (May 24, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced preliminary data from the SHUNT-V Pivotal study in oral and poster presentations at Digestive Disease Week® (DDW) 2022 in San Diego….
Chris Jensen Joins Business Advisory Board
Former J.P. Morgan Investment Banker Brings Mid-Market Capital-Raising Experience to Board DENVER (May 10, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that former J.P. Morgan Investment Banker Chris Jensen has…
Bobbi Coffin Named to Business Advisory Board
Biodesix Exec and Former Exact Sciences Commercial Leader Adds Marketing Depth to Board Denver, CO – May 5, 2022 – HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that Bobbi Coffin, has joined the company’s…
Hepion Pharmaceuticals Announces Clinical Collaboration with HepQuant in Phase 2b NASH Trial
Pharma and Medical Device Veteran Ally Xu Named Director of Regulatory and Quality Affairs
HepQuant Names Pharma and Medical Device Veteran Ally Xu Director of Regulatory and Quality Affairs DENVER (Feb. 1, 2022) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that pharmaceutical and medical device industry veteran…
Preliminary data from the HepQuant SHUNT-V Pivotal study released during the 72nd AASLD Annual Meeting
HepQuant Results Presented while Attending the 72nd AASLD Annual Meeting and AHA 2021 Annual Meeting DENVER (November 16th, 2021) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that preliminary data from the SHUNT-V Pivotal study…
Perspectum and HepQuant Announce Partnership to Bring a More Complete Picture of Liver Health
Perspectum and HepQuant Announce Partnership to Bring a More Complete Picture of Liver Health Partnership Combines Imaging and Functional Assessment for Clinical Research San Francisco, CA and Denver, CO – Nov. 1, 2021. Perspectum and HepQuant are proud to announce a strategic business partnership to provide seamless delivery of HepQuant’s…
HepQuant’s Greg Everson, MD, to Speak at AASLD/FDA Conference on Drug-Induced Liver Injury (DILI)
HepQuant’s Greg Everson, MD, to Speak at AASLD/FDA Conference on Drug-Induced Liver Injury (DILI) DENVER (April 14th, 2021) — HepQuant, LLC, a Denver, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that it will present at the 2021 AASLD/FDA Conference…
HepQuant Promotes Sean Bundy to Chief Operating Officer
HepQuant Promotes Sean Bundy, RAC, to Chief Operating Officer Denver, CO – June 10, 2020 – HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced the promotion of Sean Bundy, RAC, to the…
HepQuant to Offer Mobile and Remote Test Administration via CVS Health’s Coram Clinical Trials
HepQuant to Offer Mobile and Remote Test Administration in Clinical Trials via CVS Health’s Coram Clinical Trials DENVER (February 18th, 2020) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending blood-based technology for evaluating the function of the liver, today announced that it will now offer…
HepQuant to Present at Biotech Showcase™ 2020
HepQuant to Present at Biotech Showcase™ 2020 San Francisco Event to be held January 13–15th DENVER (Jan. 10, 2020) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending blood-based technology for evaluating the function of the liver, today announced that it has scheduled a company presentation at…
HepQuant’s Disease Severity Index (DSI) Demonstrates Hepatic Functional Improvement in High Percentage of NASH Patients Treated with Obeticholic Acid: Results from INTERCEPT 747-117
HepQuant’s Disease Severity Index (DSI) Demonstrates Hepatic Functional Improvement in High Percentage of NASH Patients Treated with Obeticholic Acid: Results from INTERCEPT 747-117 The First HepQuant SHUNT™ Data From An Interventional Drug Study in NASH DENVER (April 4, 2019) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique,…
Hoffman Joins HepQuant as Clinical Lab Manager
Keith Hoffman Joins HepQuant as Clinical Lab Manager Brings more than 20 years of pharmaceutical industry experience in LC/MS analytics and lab management Keith Hoffman DENVER (Feb. 20, 2019) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients…
First Patient Enrolled in a Pivotal Trial of the HepQuant SHUNT Liver Diagnostic Test
First Patient Enrolled in a Pivotal Trial of the HepQuant SHUNT Liver Diagnostic Test Study Aims to Enroll 420 Subjects in 20 U.S. Research Sites DENVER (Feb. 14, 2019) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients…
Sarah Downing Joins HepQuant as Senior Manager of Quality Affairs
Sarah Downing Joins HepQuant as Senior Manager of Quality Affairs Certified Quality Engineer Brings More than 14 Years Experience in Medical Device and Diagnostics Quality Affairs Sarah Downing, Sr. Manager of Quality Affairs DENVER (Nov. 15, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending…
Receives IDE Approval from U.S. FDA to Initiate Clinical Trial of the SHUNT Liver Diagnostic Kit
HepQuant Receives IDE Approval from U.S. FDA to Initiate Clinical Trial of the SHUNT Liver Diagnostic Kit SAN FRANCISCO (Nov. 9, 2018) – While attending The Liver Meeting® 2018, HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic…
Sean Bundy Appointed Director of Regulatory and Quality Affairs
HepQuant Appoints Sean Bundy Director of Regulatory & Quality Affairs Bringing 20 Years in Medical Device and Diagnostics Regulatory and Quality Experience DENVER (Aug. 2, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease,…
HepQuant Chosen to Advance to InnoSTARS Semifinals in China
HepQuant Selected as a Semifinalist for 2018 InnoSTARS Competition to be Held in China DENVER (May 9, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, announced today that the company has been named…
HepQuant a Finalist for InnoSTARS Denver Competition
HepQuant a Finalist for 2018 InnoSTARS Denver Competition DENVER (April 24, 2018) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, announced today that the company has been named a finalist for the 2018 InnoSTARS Denver…
HepQuant and CTI Clinical Trial and Consulting Services Announce First Patient Enrolled in Liver Disease Diagnostic Trial
HepQuant and CTI Clinical Trial and Consulting Services Announce First Patient Enrolled in Liver Disease Diagnostic Trial FOR IMMEDIATE RELEASE [Covington, KY ~ January 25, 2018] HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease…
HepQuant to Present Poster as Finalist at RESI San Francisco Innovation Challenge
HepQuant to Present Poster as a Finalist in the RESI San Francisco Innovation Challenge 2018 DENVER (January 2nd, 2017) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced it will present a…
HepQuant to Present at the 10th Annual Biotech Showcase
HepQuant to Present at 10th Annual Biotech Showcase Senior Executives Will Also Attend the J.P. Morgan Healthcare Conference DENVER (December 13th, 2017) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced it…
HepQuant Presents at Rocky Mountain Life Science Investor and Partnering Conference
HepQuant Presents at the 2017 Rocky Mountain Life Science Investor and Partnering Conference DENVER (October 12th, 2017) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced it presented at the 2017 Rocky Mountain…
HepQuant holding its 2017 Scientific Advisory Board Meeting in Washington D.C. on Saturday, October 21st
HepQuant to Hold 2017 Scientific Advisory Board October 21st in Washington, DC DENVER (August 31, 2017) — HepQuant, LLC, a diagnostics company, located in Greenwood Village, CO with unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced today announced it will be holding…
HepQuant to Sponsor its First Clinical Study
HepQuant to Sponsor its First Clinical Study Utilizing 3 Investigational Device Exemptions (IDEs) in Clinical Study of Patients with Alcoholic Hepatitis, NASH F3, NASH F4 DENVER (Sep. 28, 2017) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients…
HepQuant Announces Approval of 4th IDE
HepQuant Announces Approval of Fourth Investigational Device Exemption Application HepQuant SHUNT™ Liver Diagnostic Kit for use in a clinical study in subjects with Alcoholic Hepatitis DENVER (September 14, 2017) — HepQuant, LLC, a Greenwood Village, Colorado-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with…
HepQuant Completes Third State-Sponsored Grant
HepQuant Completes Third State-Sponsored Grant State of Colorado funding enables advancement of supply chain, regulatory advancements and HepQuant laboratory DENVER (August 31, 2017) — HepQuant, LLC, a diagnostics company, located in Greenwood Village, CO with unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease,…
Test Inventor and Founder Dr. Everson Accepts Role as CEO
HepQuant Test Inventor and Founder Doctor Gregory T. Everson Accepts Position of CEO DENVER (July 1, 2017) — HepQuant, LLC, a Greenwood Village, Colo.-based company with a unique, patented and patent-pending technology for evaluating the liver in patients with chronic liver disease, today announced that its liver test inventor, Dr. Greg…
Dr. Steve Helmke Appointed Chief Scientific Officer
HepQuant Announces Approval of 3rd IDE
HepQuant Announces Approval of 2nd IDE
HepQuant Announces Approval of 1st IDE
2025 Texas Society of Gastroenterology and Endoscopy Annual Meeting
Global Hepatitis Summit 2025
HepQuant’s Dr. Joanne Imperial, Chief Medical Officer, will be attending the Global Hepatitis Summit 2025 in Los Angeles and presenting a poster titled HepQuant DuO™ for Quantifying Liver Function and Physiology in the Clinic: Diagnostic Performance for Esophageal Varices in Patients with Treated and Active Chronic Hepatitis C, poster P-187.
5th Annual Liver Connect Conference
HepQuant will participate in the 5th Annual Liver Connect Conference in San Antonio, TX. A silver sponsor of the event, the HepQuant team may be found at booth 17 in the exhibit hall. Three posters submitted by HepQuant will be featured during the conference. Poster 47: Initial Clinical Experience with…
AASLD, The Liver Meeting
HepQuant is excited that six abstracts, will be presented, three of which were deemed a Poster of Distinction, at The Liver Meeting 2024 on 11/16/24 and 11/17/24 linked below. Poster of Distinction: The Oral Cholate Challenge Test Defines Likelihood of Large Esophageal Varices in an Overweight and Obese US Population …